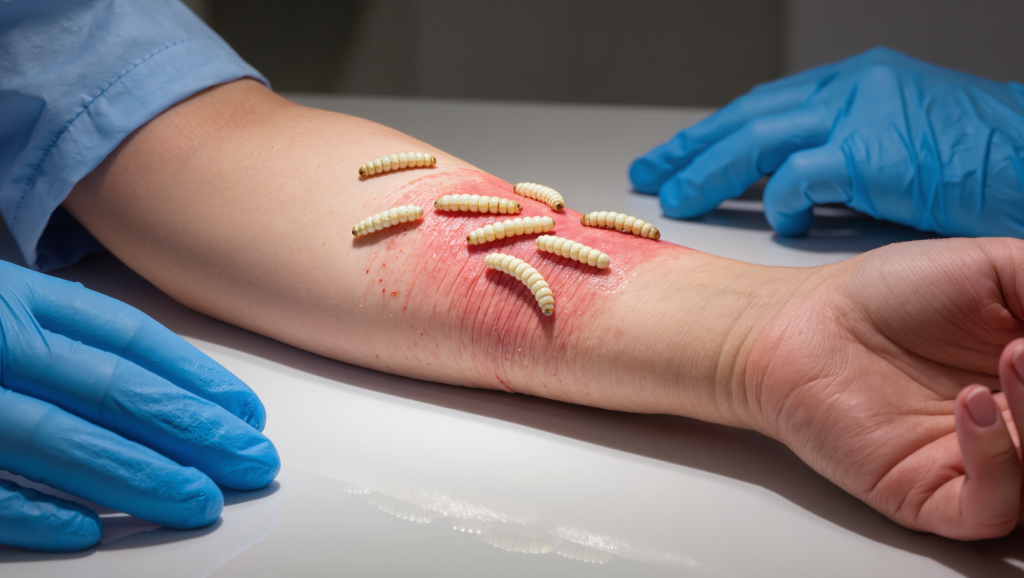
If you’ve ever seen a chronic wound that just wouldn’t heal—black, sloughy tissue, foul smell, endless dressings—you can understand why surgeons sometimes reach for the scalpel. But there’s another option that sounds like something out of a horror film and behaves like a miracle: sterile blowfly larvae that clean wounds more precisely than a surgeon’s blade. These “medical maggots” don’t just eat dead tissue; they remodel the wound micro‑environment, fight infection, and jump‑start healing in ways modern science is still mapping.
In wound‑care clinics today, maggot debridement therapy (MDT) is FDA‑cleared, reimbursable, and backed by systematic reviews—yet most people still only know it as a wartime anecdote. Looking closely at how maggots heal wounds is like watching nature’s tiny surgeons at work: they selectively dissolve necrosis, spare living tissue, disrupt bacterial biofilms, and quietly reprogram inflammation at the cellular level.
Here’s how they do it—and when they really can outperform the knife.
From Battlefield Accident to FDA‑Approved “Medical Device”
Using maggots to treat wounds is not a TikTok trend; it’s at least a century old in Western medicine and much older in folk practice.
- Surgeons in World War I and the American Civil War noticed that soldiers whose wounds were naturally infested with certain fly larvae often had less infection and cleaner tissue than expected.
- In the 1920s–30s, controlled “maggot therapy” became a legitimate hospital treatment for osteomyelitis and chronic ulcers, before antibiotics pushed it into the background.
- As antibiotic resistance and chronic wounds exploded in the late 20th century, clinicians revisited MDT. In 2004 the U.S. Food and Drug Administration cleared medicinal maggots as a “medical device” for debriding chronic, non‑healing wounds like pressure ulcers, venous stasis ulcers, diabetic foot ulcers, and non‑healing traumatic or postsurgical wounds.
The Medicinal Maggots brand (Lucilia sericata larvae) was specifically cleared “for debriding nonviable tissue in chronic wounds,” which means the FDA accepted that these larvae reliably and safely clean wounds.
Major reviews now describe MDT as efficacious, well tolerated, and cost‑effective and note that AMA and CMS reimbursement codes have opened the door to wider clinical use.
Where Maggots Shine: Chronic Wounds That Outlast Surgery
Chronic wounds—especially in diabetes, vascular disease, and spinal cord injury—are notoriously hard to treat. They often have: thick necrotic slough, tenacious biofilms, poor blood supply, and patients who can’t tolerate repeated surgery or aggressive debridement.
That’s where MDT tends to outperform standard care.
Faster, more complete debridement
A large prospective study of 435 patients with chronic wounds treated with MDT reported:
- Complete debridement in 82.1% of cases,
- Partial debridement in 16.8%,
- Ineffective treatment in only 1.1%.
Most patients needed a median of just two MDT sessions over a median of 3 treatment days to achieve results.
A 2020 systematic review comparing maggots with conventional dressings (like hydrogels) concluded that MDT:
- Achieved faster and more effective debridement of non‑viable tissue.
- Produced faster development of granulation tissue (new healthy tissue) and a greater reduction in wound surface area compared with hydrogel dressings.
- Showed no serious adverse effects, suggesting a good safety profile.
In one randomized trial of 140 patients (70 MDT vs 70 hydrogel) with venous leg ulcers, the maggot‑treated wounds had significantly more granulation tissue (p < 0.001) and smaller wound size (p < 0.05) after just 10 days.
In spinal cord injury patients with chronic pressure ulcers, standard care for 3–4 weeks achieved less than 50% debridement in all wounds, while subsequent MDT achieved complete debridement in about 10 days on average.
When surgery isn’t possible—or isn’t enough
The same review notes that MDT “should be considered a reasonable alternative in patients with chronic wounds and a primary option for those who are not fit for surgical operation or are in low‑resource settings.” It doesn’t replace surgery in all cases, but it provides:
- Repeatable “maintenance debridement” between surgical sessions.
- A way to clean wounds in frail, comorbid patients who can’t tolerate anesthesia.
- A low‑tech but biologically sophisticated option where advanced dressings or OR time are limited.
Pain and discomfort occur in some patients—one study reported increased pain or discomfort during MDT in 38%—but serious complications are rare when properly managed.
The Three Big Mechanisms Of How Maggot Therapy Works
Modern research summarises maggot action into three primary mechanisms: debridement, disinfection, and stimulation of healing.
1. Precision Debridement: Liquefying Dead Tissue, Sparing Living Cells
The greenbottle fly larva Lucilia sericata is exquisitely evolved to eat decaying flesh—not living tissue. That selectivity is MDT’s superpower.
- Maggots secrete proteolytic enzymes (proteases) into the wound, which liquefy necrotic slough and fibrin into an easily ingestible soup.
- In vitro work shows maggot excretions/secretions (ES) contain at least three classes of proteolytic enzymes: metalloproteinases, aspartyl proteases, and serine proteases (trypsin‑like and chymotrypsin‑like).
- Chymotrypsin‑like serine proteases are especially effective at dissolving fibrin clots and degrading extracellular matrix proteins like fibronectin, laminin, and type I and III collagen—the components that hold necrotic tissue together.
By dissolving necrotic tissue and fibrin nets while leaving viable tissue largely intact, maggots can clean irregular wound surfaces more gently and thoroughly than many mechanical methods.
Clinically, that translates into a wound bed that’s:
- Free of yellow/black slough.
- Covered instead with bright red granulation tissue (the “raspberry” look clinicians love).
That’s why maggots are sometimes called “living scalpels”—but they’re really more like living enzyme pumps with built‑in sensors that stop where life begins.
2. Disinfection: Crushing Bacteria and Biofilms
Chronic wounds are rarely just “dirty”—they are often colonised by complex bacterial biofilms that resist antibiotics and immune clearance. MDT has multiple anti‑microbial angles:
- Maggot secretions have direct antibacterial components; lab studies show activity against pathogens like Staphylococcus aureus and Pseudomonas aeruginosa.
- ES disrupt bacterial biofilms—the slime‑layer matrices that bacteria form—which helps restore antibiotic sensitivity and immune access.journals.
- By consuming liquefied necrotic tissue, maggots remove the nutrient‑rich substrate that bacteria feed on.
A 2018 review of the “pharmacological properties of the medical maggot” calls maggots a “miraculous medicinal” organism with antimicrobial, antibiofilm, anti‑inflammatory, and wound‑healing activities, driven largely by compounds in their secretions.
3. Stimulating Healing: Modulating Immunity and Promoting Tissue Growth
Perhaps the most surprising finding is that maggots don’t just clean; they also reprogram the wound environment to be less inflammatory and more regenerative.
Studies show that maggot excretions/secretions (ES) can:
- Down‑regulate pro‑inflammatory cytokines (like IL‑12p40, TNF‑α, and MIF) from activated immune cells, while increasing anti‑inflammatory IL‑10.
- Inhibit neutrophil and monocyte chemotaxis and reduce expression of adhesion molecules (CD11b/CD18), dampening excessive inflammatory cell infiltration.
- Inhibit complement activation (C3 and C4) in patient sera via multiple pathways, reducing complement‑mediated inflammation.
On the tissue side, those proteases don’t just debride; they also influence:
- PAR‑mediated activation of cells (protease‑activated receptors) that can stimulate proliferation and cytokine release in the wound bed.
- Liberation of growth factors and matrix fragments that promote granulation tissue formation and angiogenesis.
A mechanistic review concludes that laboratory studies and some small clinical trials “strongly suggest that maggots do promote tissue growth and wound healing, though it is likely only during and shortly after the period when they are present on the wound.”
That’s why experts propose using MDT not just to “get it clean and stop,” but as “maintenance debridement” to keep the wound environment optimally tuned for healing.
Modern Spin‑Offs Of Maggot Therapy: Bottling Maggot Saliva
Once researchers realised how powerful maggot secretions were, they began isolating specific molecules.
A 2023 study reported on a recombinant proteolytic enzyme isolated from maggot saliva with fibrinolytic action. This enzyme showed promise as a safe, effective enzymatic debridement agent in preclinical pharmacology and toxicology models, suggesting a future where we use maggot‑derived biotech without live larvae.
Still, the “whole maggot” approach delivers a complex cocktail of enzymes, antimicrobials, and immunomodulators in a self‑renewing package that’s hard to fully replicate synthetically.
What a Maggot Therapy Session Actually Looks Like
For patients, MDT is surprisingly structured and controlled.
- Species & sterility: Only disinfected eggs of Lucilia sericata (the greenbottle fly) are used, hatched under sterile conditions into “medical maggots.”
- Application methods: Larvae are applied directly to the wound (free‑range) under a special mesh dressing, or contained within “bio‑bags” (small porous pouches) that allow secretions to contact the wound without visible crawling.
- Dosing: Typical prescriptions use a certain number of larvae per square centimetre of wound area, left in place for 48–72 hours.
- Duration: Many chronic wounds need 1–3 cycles of MDT, though some studies report up to several weeks of repeated applications for very severe cases.
Patients often report:
- Sensation ranging from mild tickling to discomfort or pain—especially as the wound is cleaned and nerve exposure increases.
- Relief as odour decreases and the wound looks visibly cleaner over days.
Clinically, MDT is often combined with:
- Modern dressings (foam, hydrocolloid) between cycles.
- Off‑loading, compression, and systemic optimisation (glycaemic control, vascular interventions) for underlying causes.
MDT isn’t a stand‑alone cure, but a potent tool within comprehensive wound care.
When Maggot Therapy “Beats” Surgery—and When It Doesn’t
The title claim—“faster than surgery”—is partly metaphor, partly situational.
Where MDT tends to win:
- Deeply cavitated or irregular wounds where scalpels and curettes struggle to reach pockets of necrosis.pmc.ncbi.nlm.nih+1
- Patients unfit for repeated OR debridement due to comorbidities, anesthesia risk, or poor access.pubmed.ncbi.nlm.nih+1
- Situations where repeated gentle debridement over days is safer than aggressive one‑time cutting.
Controlled trials show that maggot therapy can achieve complete debridement in days to weeks, compared with many weeks of conventional dressings and incomplete results.
Where surgery still leads:
- Acute traumatic or necrotising infections that require immediate, radical debridement.
- Situations requiring removal of structurally compromised tissue (bone, tendon) or reconstructive planning.
In practice, many centres use MDT as a complement to surgery, not a replacement: debriding between OR sessions, or finishing what the scalpel couldn’t reach.
Safety and the Future of Maggot Therapy
Despite good data, MDT still fights an image problem—understandably.
Safety profile:
- Systematic reviews report no serious adverse events, with pain/discomfort as the most common issue, often manageable with analgesia or shorter treatment periods.
- Larvae are species‑specific, sterile, and removed after each cycle; they do not become flies in the wound.
Stigma:
- Many patients and even clinicians must overcome a “gross factor” to accept MDT. Once they see rapid debridement and wound improvement, resistance often fades.
Future directions:
- Standardised guidelines on larvae number, dressing types, and duration.journals.
- Isolated maggot‑derived enzymes (like the recombinant fibrinolytic agent) as topical drugs.
- Deeper exploration of maggot‑induced immune modulation as a model for regenerative therapies.
In other words: we’re just beginning to understand how sophisticated these tiny surgeons really are.
Takeaway: Nature’s Version of Regenerative Surgery
When you strip away the “ick,” maggot therapy is a brilliantly elegant solution:
- It uses a living tool that automatically seeks necrotic tissue, dissolves it enzymatically, spares living structures, and constantly adjusts as the wound changes.
- It brings built‑in antimicrobial and antibiofilm actions, vital at a time of rising antibiotic resistance.
- It actively recalibrates inflammation and promotes granulation, nudging chronic, stalled wounds back into a healing trajectory.
Surgery will always have its place. But for many stubborn, slough‑filled wounds, these tiny, wriggling “surgeons” can do what scalpels and dressings often cannot: turn a rotting crater into a clean, red, healing wound in a matter of days—quietly, cheaply, and with a precision that’s hard not to call anything but genius.
Sources: